Research Content
1. Silsesquioxane
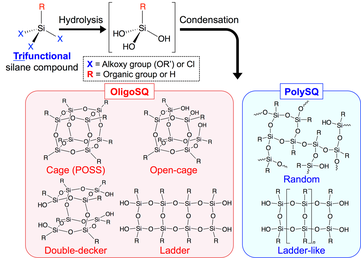
Silsesquioxanes (SQs; general formula: [RSiO1.5]n) are a class of siloxane-based materials that are generally prepared by the hydrolytic condensation of trifunctional silane compounds, such as organotrialkoxy- and organotrichlorosilanes (Scheme 1). SQs have attracted much attention in organic–inorganic hybrid material research for academic and practical reasons, because they are inorganic materials with various functional organic side chains (R) and have remarkable compatibility with organic materials, such as polymers. In addition they exhibit superior thermal, mechanical and chemical stabilities derived from the siloxane (Si-O-Si) framework, which has a high bond energy.
2. Rod-like and Ladder-like Polysilsesquioxanes (PSQs)
Among the SQs, some polymeric SQ (PSQ) compounds with regular structures, such as ladder-like PSQs, are soluble in aqueous/organic media and have the ability to form films. However,
although various types of polyhedral oligomeric SQs (POSSs) are known as regularly structured SQs, ladder-like PSQs have only been obtained in the limited cases, because PSQs are prepared by the
polycondensation of trifunctional silane monomers. These monomers generally result in the formation of POSS compounds or insoluble PSQs with irregular three dimensional networks (random)
structures.
2-1. Preparation of ammonium-group-containing rod- and ladder-like PSQs with hexagonally stacked structures

To date, we have successfully prepared soluble ammonium-group-containing rod- and ladder-like PSQ (PSQ-NH3Cl) with a hexagonally stacked structure by hydrolytic polycondensation of amino-group-containing organotrialkoxysilane (APTMS) using aqueous solutions of strong acids, e.g., HCl, as catalysts (Scheme 2-1) [1-5].
When the hydrolytic polycondensation of APTMS was performed under alkaline conditions using NH3 aqueous solution, insoluble PSQ with three-dimensional network structure was prepared. Therefore, we considered that self-organization of the ions prepared from the amino group of APTMS and the strong acid was the driving force for the formation of regular structures of the PSQ.
[1] Y. Kaneko, N. Iyi, K. Kurashima, T. Matsumoto, T. Fujita, and K. Kitamura, Chem. Mater., 2004, 16, 3417. URL
[2] Y. Kaneko, N. Iyi, T. Matsumoto, and K. Kitamura, Polymer, 2005, 46, 1828. URL
[3] Y. Kaneko and N. Iyi, Z. Kristallogr., 2007, 222, 656. URL
[4] Y. Kaneko, H. Toyodome, M. Shoiriki, and N. Iyi, Int. J. Polym. Sci., 2012, 684278. URL
[5] Y. Kaneko, Kobunshi Ronbunshu (Japanese), 2014, 71, 443. URL
2-2. Preparation of carboxylate-group-containing rod- and ladder-like PSQ with hexagonally stacked structure
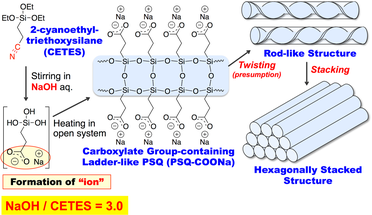
Carboxylate-group-containing rod- and ladder-like polysilsesquioxane (PSQ-COONa) with a hexagonally stacked structure was successfully prepared by the hydrolytic polycondensation of 2- cyanoethyltriethoxysilane (CETES) monomer in sodium hydroxide aqueous solution (Scheme 2-2) [6]. Self-organization of ions composed of a carboxylate anion and a sodium cation, which was converted from the cyano group of CETES by hydrolysis under alkaline conditions, was a driving force for the formation of such regular structures of PSQ-COONa.
[6] H. Toyodome, Y. Kaneko, K. Shikinaka, and N. Iyi, Polymer, 2012, 53, 6021. URL
2-3. Preparation of sulfo-group-containing rod- and ladder-like PSQ with hexagonally stacked structure
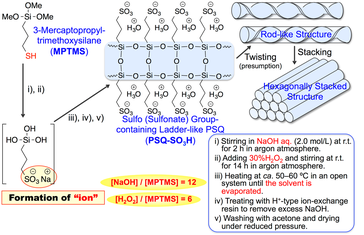
A sulfo-group-containing rod-like polysilsesquiox- ane with a hexagonally stacked structure (PSQ-SO3H) was successfully prepared by oxidation and hydrolytic polycon- densation of 3-mercaptopropyl-trimethoxysilane (MPTMS) in a mixed aqueous solution of NaOH and H2O2 (Scheme 2-3) [7].
The presence of ionic side-chains composed of the sulfonate anions and sodium cations during the hydrolytic polycondensation of MPTMS was found to be essential for the formation of this regularly structured PSQ. Finally, the proton conductivity of the PSQ-SO3H film was relatively high (> 10^-2 S/cm).
[7] Y. Kaneko, H. Toyodome, T. Mizumo, K. Shikinaka, and N. Iyi, Chem. Eur. J., 2014, 20, 9394. URL
3. Cage-like Oligosilsesquioxanes (POSSs)
Polyhedral oligomeric silsesquioxanes (POSSs), which can be prepared by the hydrolytic condensation of trifunctional silane compounds, have attracted much attention from both academia and industry because of their many useful applications. In particular, POSSs containing reactive organic groups are more attractive in materials research because the reactive groups enable covalent bonding of the POSS to polymers, resulting in the development of polymer-related organic-inorganic hybrid materials.
3-1. Preparation of cage-like oligo(3-aminopropyl)silsesquioxane (POSS) in higher yield with a shorter reaction time
Cage-like oligo(3-aminopropyl)-silsesquioxane (AP-POSS) is one of the useful reactive POSS compounds and can be employed as a precursor for the preparation of various functional POSS
derivatives. AP-POSS was first prepared by the hydrolytic condensation of 3-aminopropyl-trialkoxysilane under moderate reaction conditions (i.e., using a mixed solvent of MeOH with conc.
HCl at 25 degrees C) in relatively low yield (ca. 30%) and requiring a long reaction time (over a period of 6 weeks) [1]. Afterward, it was reported that OAP-POSS could be obtained in
almost the same yield (ca. 30%) with a shorter reaction time (5–10 days) compared with the aforementioned process by optimization of the reaction conditions [2].
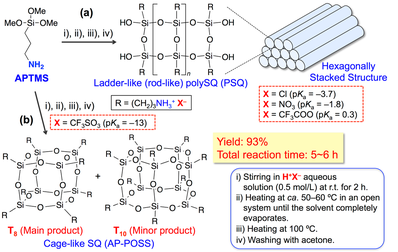
As described above, we have found that a soluble ladder-like PSQ with a hexagonally stacked structure could be quantitatively prepared by the hydrolytic condensation of APTMS in aqueous inorganic acids, such as HCl and HNO3 (Scheme 3-1(a)).
In this study, we investigated the hydrolytic condensation of APTMS using various acid catalysts under the same reaction conditions as those used for the preparation of the ladder-like PSQ. Consequently, we found that AP-POSS was prepared in higher yield (ca. 93%) with a shorter reaction time (ca. 5–6 h) using the superacid trifluoromethanesulfonic acid (CF3SO3H) as the catalyst (Scheme 3-1(b)) [3].
[1] F. J. Feher and K. D. Wyndham, Chem. Commun., 1998, 323.
[2] M. C. Gravel, C. Zhang, M. Dinderman, and R. M. Laine, Appl. Organomet. Chem., 1999, 13, 329.
[3] Y. Kaneko, M. Shoiriki, and T. Mizumo, J. Mater. Chem., 2012, 22, 14475. URL
3-2. Preparation of low-crystalline POSS containing two types of alkylammonium groups
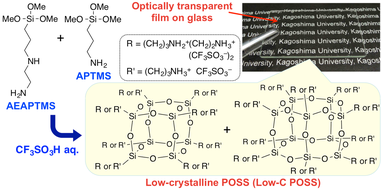
In this study, a low-crystalline POSS (Low-C POSS) containing two types of alkylammonium groups was successfully prepared by hydrolytic condensation of a mixture of two types of
amino group-containing organotrialkoxysilanes, i.e. 3-(2-aminoethylamino)-propyltrimethoxysilane (AEAPTMS) and APTMS, using aqueous CF3SO3H as the catalyst and solvent (Scheme 3-2)
[4]. Due to the low molecular symmetry of the resulting POSS compound containing two different randomly distributed side-chain groups, its crystallization was suppressed, leading to the formation
of an optically transparent film.
[4] T. Tokunaga, M. Shoiriki, T. Mizumo, and Y. Kaneko, J. Mater. Chem. C, 2014, 2, 2496. URL
3-3. Facile preparation of a soluble polymer containing POSS units in its main chain
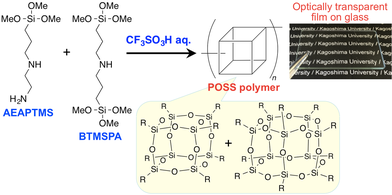
A soluble polymer containing POSS units in its main chain was successfully prepared in one-step by hydrolytic condensation of a mixture of two types of amino group-containing organotrialkoxysilanes using the aqueous CF3SO3H as a catalyst (Scheme 3-3) [5].
In this CF3SO3H-catalysed synthesis, AEAPTMS and bis[3-(trimethoxysilyl)propyl]amine (BTMSPA) acted as a starting material of POSS and a cross-linker, respectively.
The weight-average molecular weight was 3.24 × 10^4. The POSS polymer provided optically transparent films. This may result from the coexistence of POSS components bearing two different
randomly distributed side-chain groups in the polymer, which suppresses crystallization. The POSS polymer exhibited 5% and 10% weight losses at 351 °C and 368 °C, respectively, indicative of its
relatively high thermal stability.
[5] T. Tokunaga, S. Koge, T. Mizumo, J. Ohshita, and Y. Kaneko, Polym. Chem., 2015, 6, 3039. URL
4. Ionic Liquids Containing Silsesquioxane Frameworks
Ionic liquids, which are molten salts below 100°C, have been widely studied for their remarkable potential as reaction solvents, extraction solvents, and electrolyte materials,
because they exhibit superior properties such as negligible vapor pressure, high thermal stability, and high ionic conductivity. Ionic liquids consist of organic cations with either organic or
inorganic anions. Most ionic liquids are regarded as organic compounds because of the presence of at least one organic ion. On the other hand, it is generally difficult to prepare ionic liquids
containing inorganic frameworks.
Recently, Chujo et al. developed an ionic liquid containing POSS as the inorganic component, which had carboxylate anion side chains and imidazolium cations as counter ions, by a multistep reaction pathway [1]. Because the POSS component has a siloxane bond framework, this compound exhibits relatively high thermostability compared with an ionic liquid compound having the structure of the side chains of this POSS.
[1] K. Tanaka, F. Ishiguro, Y. Chujo, J. Am. Chem. Soc., 2010, 132, 17649.
4-1. Preparation of trimethylpropylammonium salt-type ionic liquids containing silsesquioxane frameworks
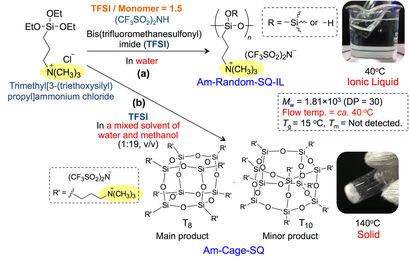
A trimethylpropyl-ammonium salt-type ionic liquid containing silsesquioxane framework (Am-Random-SQ-IL) was successfully prepared by the hydrolytic condensation of trimethyl[3-(triethoxysilyl)propyl]-ammonium chloride (TMTESPAC) in aqueous solution of bis(trifluoromethane-sulfonyl)imide (TFSI) (Scheme 4-1(a)) [2]. On the basis of the results of EDX, 29Si NMR, SLS, and XRD, it was concluded that Am-Random-SQ-IL was an amorphous oligomeric silsesquioxane compound containing equimolar quaternary ammonium cations and TFSI anions. The DSC thermogram of Am-Random-SQ-IL exhibited an endotherm peak due to Tg at 15 °C, and showed fluidity over ca. 35 °C, indicating that Am-Random-SQ-IL was fluid below 100 °C (i.e., an ionic liquid).
In the TG analyses, the temperatures of thermal decomposition of Am-Random-SQ-IL were higher than those of N,N,N-trimethyl-N-propylammonium bis(trifluoromethanesulfonyl)imide, which is an ionic liquid compound with the same structure as that of the side chains of Am-Random-SQ-IL. This result indicated that the silsesquioxane framework could suppress thermal degradation.
On the other hand, when the hydrolytic condensation of TMTESPAC was performed using TFSI solution in water/methanol mixed solvent (1:19 v/v) on behalf of the aforementioned aqueous solution, we found that powdered cage-like octasilsesquioxane (Am-Cage-SQ) was obtained as the main product (Scheme 4-1(b)). Am-Cage-SQ maintained its solid state at 140 °C (i.e., it was not an ionic liquid).
[2] T. Ishii, T. Mizumo, and Y. Kaneko, Bull. Chem. Soc. Jpn., 2014, 87, 155. URL
4-2. Preparation of 1-methyl-3-propylimidazolium salt-type ionic liquids containing silsesquioxane frameworks
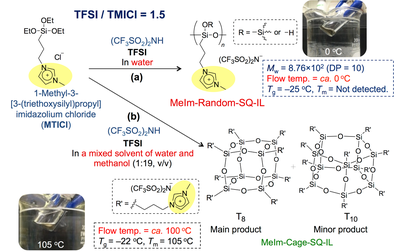
An ionic liquid containing a random-structured oligosilsesquioxane (MeIm-Random-SQ-IL) was successfully prepared by the hydrolytic condensation of 1-methyl-3-[3-(triethoxysilyl)propyl]-imidazolium chloride (MTICl) in aqueous TFSI (Scheme 4-2(a)) [3]. MeIm-Random-SQ-IL exhibited a Tg at -25 °C as indicated by an endothermic peak in the DSC curve. In addition, fluidity was visually observed at ca. 0 °C, i.e. MeIm-Random-SQ-IL is a room temperature ionic liquid.
Conversely, when the hydrolytic condensation of MTICl was performed using a water/methanol (1:19 v/v) solution of TFSI, an ionic liquid containing a cage-like oligosilsesquioxane (MeIm-Cage-SQ-IL) was obtained (Scheme 4-2(b)) [3]. The Tg of Im-Cage-SQ-IL was -22 °C, and its melting temperature (Tm) was 105 °C according to the DSC analysis. In addition, fluidity was observed for this ionic liquid at ca. 100 °C. These results suggest that both the amorphous structure of MeIm-Random-SQ-IL and the type of substituent groups in the silsesquioxane contributed to the ionic liquid behaviour below room temperature. In addition, these ionic liquids exhibited high thermal stabilities.
[3] T. Ishii, T. Enoki, T. Mizumo, J. Ohshita, and Y. Kaneko, RSC Adv., 2015, 5, 15226. URL
5. Cyclic Siloxanes
5-1. Selective synthesis of cis−trans−cis cyclic tetrasiloxanes and the formation of their two-dimensional layered aggregates
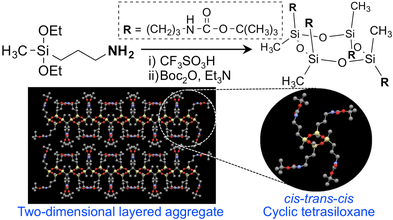
Cyclic siloxanes are well-known raw materials to prepare silicones, cosmetics, dry-cleaning solvents, skin cleansing agents, emulsifiers, etc. They are generally prepared by the
hydrolytic condensation of silane compounds containing two reactive groups and two organic groups. In the case of cyclic tetrasiloxanes obtained from silane compounds containing two different
organic groups, they stochastically have four isomers, all-cis, all-trans, cis−trans−cis, and cis−cis−trans structures.
Because it is difficult to selectively synthesize a single cyclic tetrasiloxane among these four isomers, isolation by distillation, recrystallization, and/or chromatography are generally
required to obtain such a single cyclic tetrasiloxane.
In this study, a single cyclic tetrasiloxane containing propylammonium trifluoromethanesulfonate and methyl side-chain groups (Am-CyTS) was selectively prepared by the hydrolytic condensation of 3-aminopropyldiethoxymethylsilane using aqueous superacid trifluoromethanesulfonic acid (CF3SO3H) (Scheme 5-1) [1].
The 1H NMR spectrum of Am-CyTS in D2O exhibited a single signal assigned to a methyl group, and the 29Si NMR spectrum of Am-CyTS in DMSO-d6 also exhibited only one signal. In the MALDI-TOF MS and the ESI MS analyses, the peaks corresponding to the masses of the cyclic tetrasiloxane were observed. These results indicate that Am-CyTS is a single cyclic tetrasiloxane without isomers. In addition, the result of a single-crystal X-ray structural analysis of its tert-butoxycarbonyl (Boc)-protected compound (Boc-CyTS) indicated the formation of a cis−trans−cis cyclic tetrasiloxane forming two-dimensional layered aggregates (Scheme 5-1). Moreover, it was found that two-dimensional layered aggregates could be formed by drop-casting an aqueous solution of Am-CyTS and chloroform solution of Boc-CyTS onto glass substrates, as shown by powder X-ray diffraction measurements.
[1] S. Kinoshita, S. Watase, K. Matsukawa, and Y. Kaneko, J. Am. Chem. Soc., 2015, 137, 5061. URL
Last updated on May 7, 2015
 Kaneko Laboratory
Kaneko Laboratory